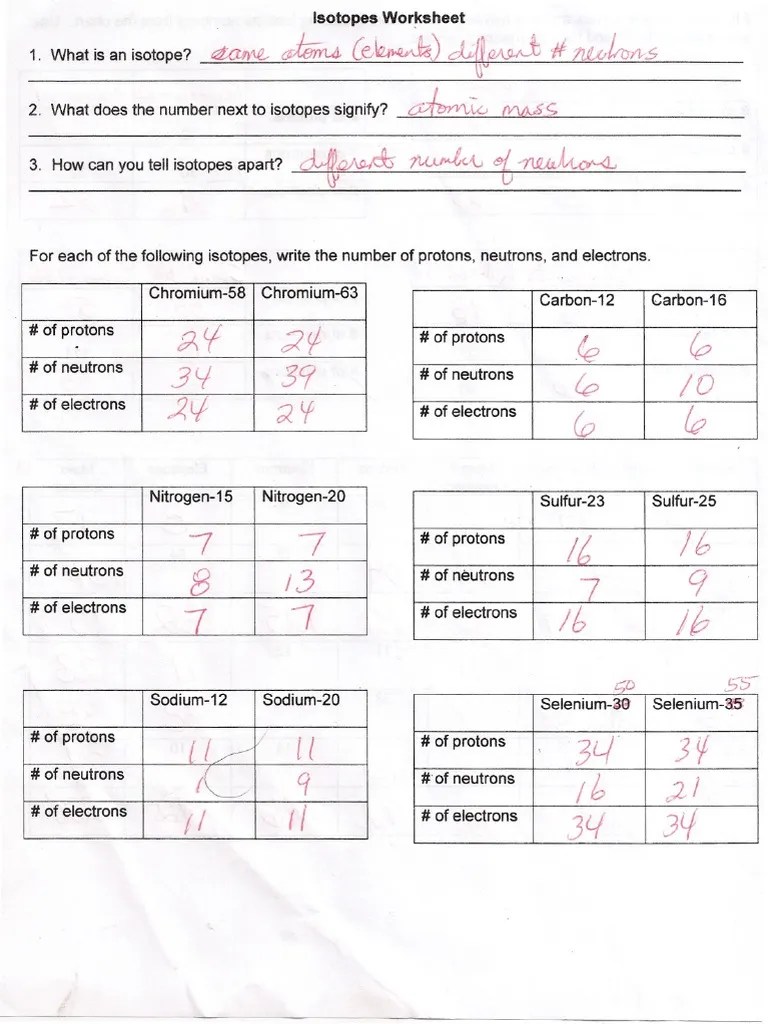
Isotopes Practice Worksheet Answer Key - 612 c 613 c 614 c a. 19 protons, 22 neutrons, 19 electrons. In a neutral atom the number of protons equals the number of electrons. 5 protons, 6 neutrons, 5 electrons. Isotopes practice set oe aa — 1. Web fill in the isotope names and any missing information, including isotope numbers from the chart. An atom can never gain or lose protons 3. The number 6 refers to the. Use your periodic table and the. Web here are three isotopes of an element:
Web here are three isotopes of an element: Isotopes practice set oe aa — 1. 19 protons, 22 neutrons, 19 electrons. In a neutral atom the number of protons equals the number of electrons. The number 6 refers to the. Web fill in the isotope names and any missing information, including isotope numbers from the chart. Use your periodic table and the. 5 protons, 6 neutrons, 5 electrons. 612 c 613 c 614 c a. An atom can never gain or lose protons 3.