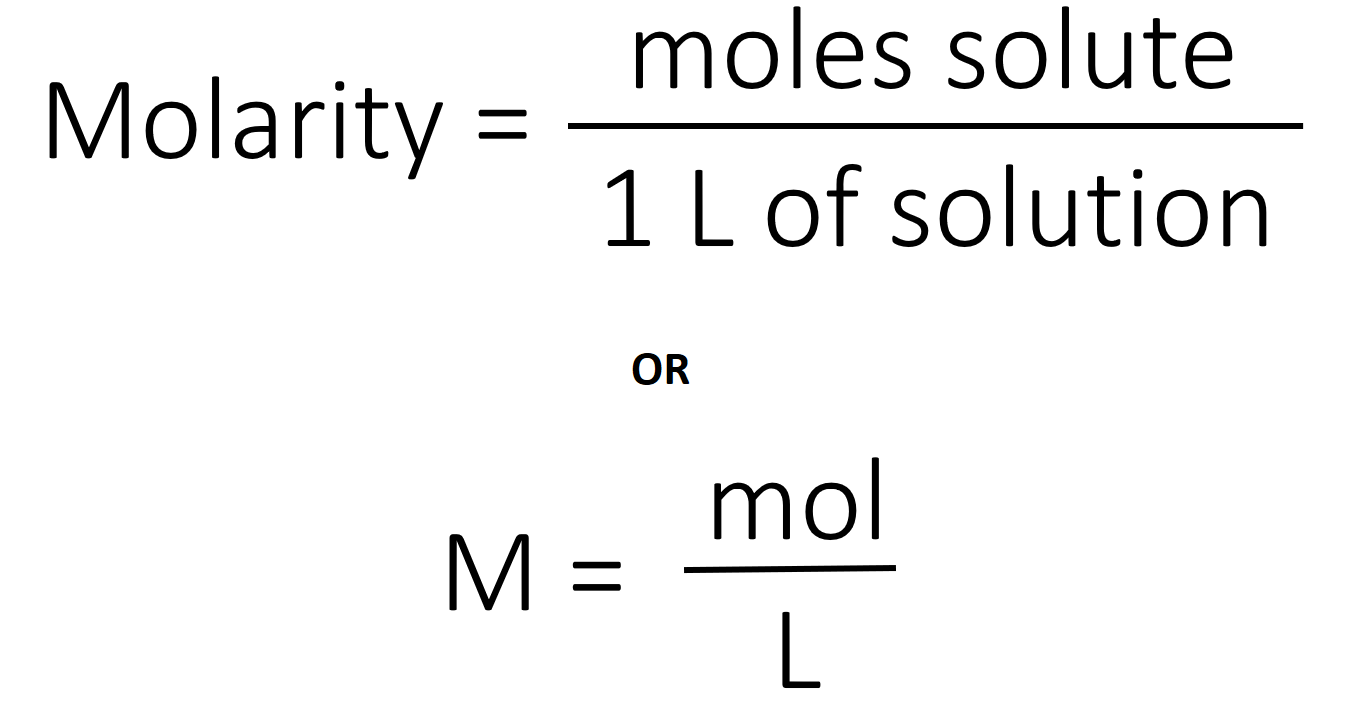Molarity Chemistry Worksheet - 1.0 mole kf = 10. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Calculate the molarity of 0.289 moles of fecl3 dissolved in 120 ml of solution? 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Web solutions what is the molarity of the following solutions given that: Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Web calculate the number of moles and the mass of the solute in each of the following solutions: In the first equation, the. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric acid.
1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. 1.0 mole kf = 10. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric acid. Web the equation for molarity states that the molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Calculate grams of solute needed to. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Web calculate the number of moles and the mass of the solute in each of the following solutions: Web solutions what is the molarity of the following solutions given that: In the first equation, the. Calculate the molarity of 0.289 moles of fecl3 dissolved in 120 ml of solution?